Methods and measuring
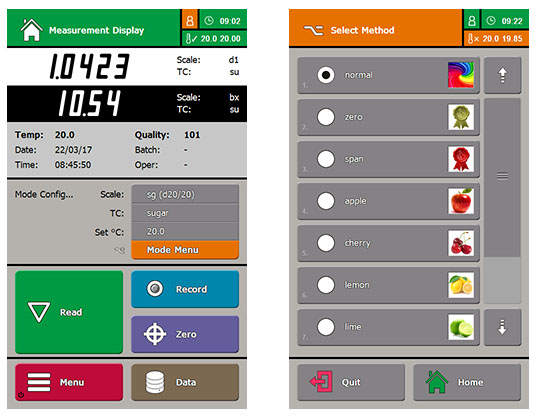 Measurements with the RFM300-T are made through a familiar measuring mode or by way of a simple Methods system with one-touch configuration of scale, temperature and data storage, with up to 8000 results being saved alongside pre-set limits that can be used as an audit tool. Methods may be user-defined or selected from the on-board library. As an example, the ‘Coffee Solids’ Method allows for coffee type and daily offsets to be inputted, so that the %TS achieved using the refractometer in the factory match the results of the laboratory tests used to configure the freeze-drying process, producing instant coffee powder.
Measurements with the RFM300-T are made through a familiar measuring mode or by way of a simple Methods system with one-touch configuration of scale, temperature and data storage, with up to 8000 results being saved alongside pre-set limits that can be used as an audit tool. Methods may be user-defined or selected from the on-board library. As an example, the ‘Coffee Solids’ Method allows for coffee type and daily offsets to be inputted, so that the %TS achieved using the refractometer in the factory match the results of the laboratory tests used to configure the freeze-drying process, producing instant coffee powder.
RFM300-T Series refractometers are supplied with free software for your PC so that when connected to a network via Ethernet connection, the instrument can be remotely handed by an external service or support centre for diagnostics or even be used for training or installation qualification. USB ports are standard and facilitate connections to peripherals including an external keyboards, barcode readers or memory sticks. Memory sticks can be used to transfer secure files or as part of a “Back-up & Clone” process that allows rapid configuration interchange across a number of instruments at a customer site or for setting up hire equipment on loan for a short period of time.
Inherent to the design of the RFM300-T Digital Refractometer is the new high definition, capacitive touch-screen display that not only facilitates operation in factory environments, even when operated whilst wearing gloves, but also serves as the centrepiece for the new software. Combined, the touchscreen and GUI help the operator quickly manoeuvre through the user and configuration menus. On-screen graphical prompts support simple operation such as method loading, calibration and routine maintenance.
RFID swipe technology comes as standard, providing clearance and a log of operator and configuration functions – especially useful when being used in FDA-regulated environments operating in accordance with 21 CFR Part 11, or where good practice is adopted, e.g. as part of HACCP.